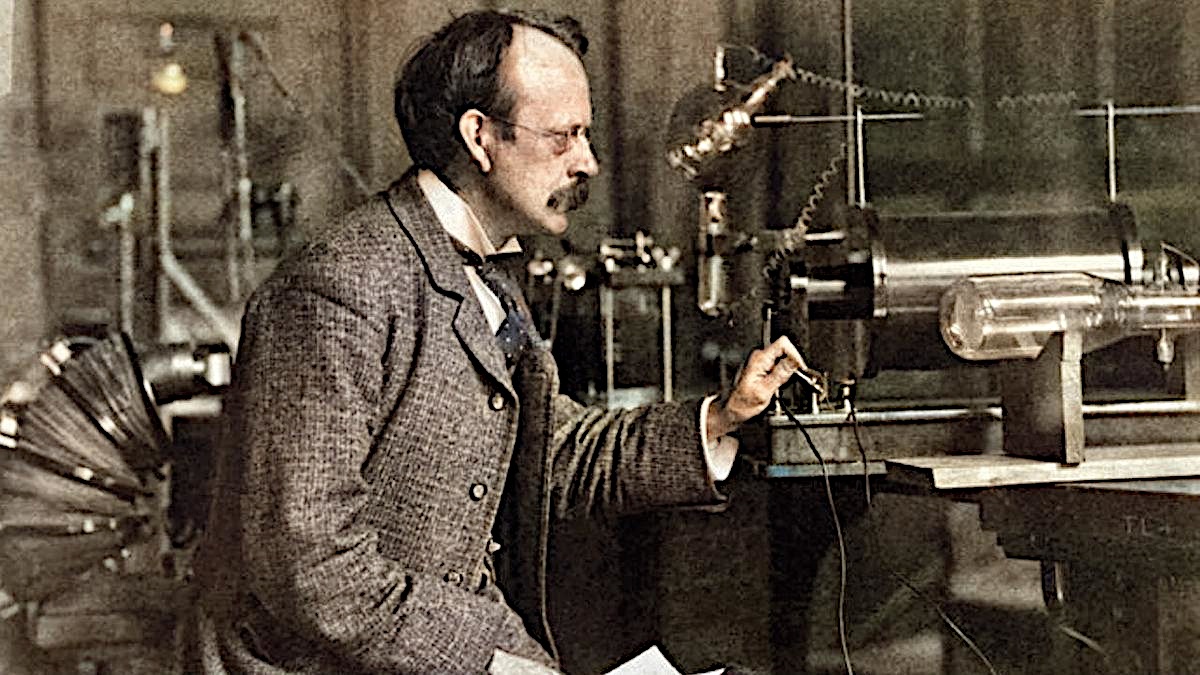Joseph John “J. J.” Thomson was a British physicist credited with discovering electrons and isotopes, for which he received a Nobel prize.
Early life
Joseph John Thomson was born in 1856 in Cheetham Hill, England Emma Swindells and Joseph James Thomson, an antiquarian bookshop keeper. Joseph James Thomson was of Scottish origin which explains the spelling of his surname. He had a brother two years younger than he, Frederick Vernon Thomson.
Education
J.J. Thomson received his early education in small private schools where he showed great interest in science followed by formidable success in that area of his education. In 1870, at the age of 14 he attended Owens College. Following the death of his father, he moved on to Trinity College, Cambridge in 1876. In 1880, he obtained his BA in mathematics in 1883. In 1884 he became Cavendish Professor of Physics.
Achievements
In 1897, Thomson was the first to suggest that the fundamental unit was over 1000 times smaller than an atom, implicating an existence of a particle today known as electron. Thomson came to this conclusion during his explorations on the properties of cathode rays. Thomson made his suggestion on 30 April 1897 following his discovery that Lenard rays could travel much further through air than expected for an atomic-sized particle. He concluded that the rays were composed of very light, negatively charged particles which were a universal building block of atoms. He called the particles “corpuscles”, but later scientists preferred the name electron. In 1906, J.J. Thomson was awarded a Nobel Prize for Physics.
Later life
In 1908 he was knighted and appointed to the Order of Merit in 1912. In 1918 he became Master of Trinity College, Cambridge, where he remained until his death. He died on August 30, 1940 and was buried in Westminster Abbey, close to Sir Isaac Newton.
Joseph John Thomson quotes
“This example illustrates the differences in the effects which may be produced by research in pure or applied science. A research on the lines of applied science would doubtless have led to improvement and development of the older methods—the research in pure science has given us an entirely new and much more powerful method. In fact, research in applied science leads to reforms, research in pure science leads to revolutions, and revolutions, whether political or industrial, are exceedingly profitable things if you are on the winning side.”